History The ancient Egyptians were the first civilization to have trained clinicians to treat physical aliments. Medical papyri, such as the Edwin Smith papyrus (circa 1600 BC) and the Ebers papyrus (circa 1534 BC), provided detailed information of management of disease, including wound management with the application of various potions and grease to assist healing.1,2 (See images below.)
The scale of wound infections was most evident in times of war. During the American Civil War, erysipelas (necrotizing infection of soft tissue) and tetanus accounted for over 17,000 deaths, according to an anonymous source in 1883. Because compound fractures at the time almost invariably were associated with infection, amputation was the only option, despite a 25-90% risk of amputation stump infection. Koch (Professor of Hygiene and Microbiology, Berlin, 1843-1910) first recognized the cause of infective foci as secondary to microbial growth in his 19th century postulates. Semmelweis (Austrian obstetrician, 1818-1865) demonstrated a 5-fold reduction in puerperal sepsis by hand washing between performing postmortem examinations and entering the delivery room. Joseph Lister (Professor of Surgery, London, 1827-1912) and Louis Pasteur (French bacteriologist, 1822-1895) revolutionized the entire concept of wound infection. Lister recognized that antisepsis could prevent infection.4 In 1867, Lister placed carbolic acid into open fractures to sterilize the wound and to prevent sepsis and hence the need for amputation. In 1871, Lister began to use carbolic spray in the operating room to reduce contamination. However, the concept of wound suppuration persevered even among eminent surgeons, such as John Hunter, 1728-1793.5 World War I (WWI) resulted in new types of wounds from high-velocity bullet and shrapnel injuries coupled with contamination by the mud from the trenches. Antoine Depage (Belgian military surgeon, 1862-1925) reintroduced wound debridement and delayed wound closure and relied on microbiological assessment of wound brushings as guidance for the timing of secondary wound closure.6 Alexander Fleming (microbiologist, London, 1881-1955) performed many of his bacteriological studies during WWI and is credited with the discovery of penicillin. As late as the 19th century, aseptic surgery was not routine practice. Sterilization of instruments began in the 1880s as did the wearing of gowns, masks, and gloves. Halsted (Professor of Surgery, Johns Hopkins University, United States, 1852-1922) introduced rubber gloves to his scrub nurse (and future wife) because she was developing skin irritation from the chemicals used to disinfect instruments. The routine use of gloves was introduces by Halsted's student J. Bloodgood. Penicillin first was used clinically in 1940 by Howard Florey. With the use of antibiotics, a new era in the management of wound infections commenced. Unfortunately, eradication of the infective plague affecting surgical wounds has not ended because of the insurgence of antibiotic-resistant bacterial strains and the nature of more adventurous surgical intervention in immunocompromised patients and in implant surgery. Wound healing is a continuum of complex interrelated biological processes at the molecular level. Healing is divided into the following phases for descriptive purposes: inflammatory phase, proliferative phase, and maturation phase. The inflammatory phase commences as soon as tissue integrity is disrupted by injury; this begins the coagulation cascade to limit bleeding. Platelets are the first of the cellular components that aggregate to the wound, and, as a result of their degranulation (platelet reaction), they release several cytokines (or paracrine growth factors). These cytokines include platelet derived growth factor (PDGF), insulinlike growth factor-1 (IGF-1), epidermal growth factor (EGF), and fibroblast growth factor (FGF). Serotonin is also released, which, together with histamine (released by mast cells), induces a reversible opening of the junctions between the endothelial cells, allowing the passage of neutrophils and monocytes (which become macrophages) to the site of injury. This large cellular movement to the injury site is induced by cytokines secreted by the platelets (chemotaxis) and by further chemotactic cytokines secreted by the macrophages themselves once at the site of injury. These include transforming growth factor alpha (TGF-alpha) and transforming growth factor beta (TGF-beta). Consequently, an inflammatory exudate that contains red blood cells, neutrophils, macrophages, and plasma proteins, including coagulation cascade proteins and fibrin strands, fills the wound in a matter of hours. Macrophages not only scavenge but they also are central to the wound healing process because of their cytokine secretion. The proliferative phase begins as the cells that migrate to the site of injury, such as fibroblasts, epithelial cells, and vascular endothelial cells, start to proliferate and the cellularity of the wound increases. The cytokines involved in this phase include FGFs, particularly FGF-2 (previously known as basic FGF), which stimulates angiogenesis and epithelial cell and fibroblast proliferation. The marginal basal cells at the edge of the wound migrate across the wound, and, within 48 hours, the entire wound is epithelialized. In the depth of the wound, the number of inflammatory cells decreases with the increase in stromal cells, such as fibroblasts and endothelial cells, which, in turn, continue to secrete cytokines. Cellular proliferation continues with the formation of extracellular matrix proteins, including collagen and new capillaries (angiogenesis). This process is variable in length and may last several weeks. In the maturation phase, the dominant feature is collagen. The dense bundle of fibers, characteristic of collagen, is the predominant constituent of the scar. Wound contraction occurs to some degree in primary closed wounds but is a pronounced feature in wounds left to close by secondary intention. The cells responsible for wound contraction are called myofibroblasts, which resemble fibroblasts but have cytoplasmic actin filaments responsible for contraction. The wound continuously undergoes remodeling to try to achieve a state similar to that prior to injury. The wound has 70-80% of its original tensile strength at 3-4 months postoperative. Surgical site infections (SSIs) are not an extinct entity; they account for 14-16% of the estimated 2 million nosocomial infections affecting hospitalized patients in the United States.7 Internationally, the frequency of SSI is difficult to monitor because criteria for diagnosis might not be standardized. A survey sponsored by the World Health Organization demonstrated a prevalence of nosocomial infections varying from 3-21%, with wound infections accounting for 5-34% of the total.8 The 2002 survey report by the Nosocomial Infection National Surveillance Service (NINSS), which covers the period between October 1997 and September 2001, indicates that the incidence of hospital acquired infection related to surgical wounds in the United Kingdom is as high as 10% and costs the National Health Service in the United Kingdom approximately 1 billion pounds (1.8 billion dollars) annually. Collated data on the incidence of wound infections probably underestimate true incidence because most wound infections occur when the patient is discharged, and these infections may be treated in the community without hospital notification. SSIs are associated not only with increased morbidity but also with mortality. Seventy-seven percent of the deaths of surgical patients were related to surgical wound infection.9 Kirkland et al calculated a relative risk of death of 2.2 attributable to SSIs, compared to matched surgical patients without infection.10 Surgical site infection is a difficult term to define accurately because it has a wide spectrum of possible clinical features. The Centers for Disease Control and Prevention (CDC) have defined SSI to standardize data collection for the National Nosocomial Infections Surveillance (NNIS) program.11,12 SSIs are classified into incisional SSIs, which can be superficial or deep, or organ/space SSIs, which affect the rest of the body other than the body wall layers. According to a report by the NNIS program,13 surgical site infections are defined as follows:
All surgical wounds are contaminated by microbes, but in most cases, infection does not develop because innate host defenses are quite efficient in the elimination of contaminants. A complex interplay between host, microbial, and surgical factors ultimately determines the prevention or establishment of a wound infection. Factors that affect surgical wound healing are classified in the chart below.
Open table in new window Table 2: Surgical Wound Classification and Subsequent Risk of Infection (If No Antibiotics Used)11,15
Open table in new window Uninfected operative wound
Elective entry into respiratory, biliary, gastrointestinal, urinary tracts and with minimal spillage
Nonpurulent inflammation present
Purulent inflammation present
Abdominal Abscess
The use of antibiotics was a milestone in the effort to prevent wound infection. The concept of prophylactic antibiotics was established in the 1960s when experimental data established that antibiotics had to be in the circulatory system at a high enough dose at the time of incision to be effective.16 General agreement exists that prophylactic antibiotics are indicated for clean-contaminated and contaminated wounds (see Table 2). Antibiotics for dirty wounds are part of the treatment because infection is established already. Clean procedures might be an issue of debate. No doubt exists regarding the use of prophylactic antibiotics in clean procedures in which prosthetic devices are inserted because infection in these cases would be disastrous for the patient. However, other clean procedures (eg, breast surgery) may be a matter of contention.17,18 Criteria for the use of systemic preventive antibiotics in surgical procedures are as follows: Qualities of prophylactic antibiotics include efficacy against predicted bacterial microorganisms most likely to cause infection, good tissue penetration to reach wound involved, cost effectiveness, and minimal disturbance to intrinsic body flora (eg, gut).19 The timing of administration is critically important because the concentration of the antibiotic should be at therapeutic levels at the time of incision, during the surgical procedure, and, ideally, for a few hours postoperatively.11 Administration of the antibiotic is by IV; 30 minutes prior to incision is the recommended time.20Antibiotics should not be administered more than 2 hours prior to surgery. Colorectal surgical prophylaxis additionally requires bowel clearance with enemas and oral nonabsorbable antimicrobial agents 1 hour before surgery.21 High-risk cesarean surgical cases require antibiotic administration as soon as the clamping of the umbilical cord is completed.11 See Table 3 for specific antibiotics recommended. Open table in new window The current risk index used to predict the risk of developing a wound infection is the NNIS system of the CDC.11The risk index category is established by the added total of the risk factors present at the time of surgery. For each risk factor present, a point is allocated; risk index values range from 0-3. This risk index is a better predictor for SSIs than the surgical wound classification (see Table 2 and Table 5).22 The NNIS risk index integrates the 3 main determinants of infection, namely, bacteria, local environment, and systemic host defenses (patient health status). The risk index does not include other risk variables, like smoking, tissue oxygen tension, glucose control, shock, and maintenance of normothermia. All these factors are relevant for clinicians but difficult to monitor and fit into a manageable risk assessment. The elements constituting this index are as follows: Open table in new window Open table in new window *Hospital Infection Control Practices Advisory Committee (HICPAC) recommendations (partial) for the prevention of SSIs, April 1999 (non–drug based) Open table in new window *Previous nomenclature of 1992 CDC guidelines Preoperative patient preparation Preoperative considerations for surgical team members Preoperative and postoperative wound care
Theater environment and care of instrumentation
Special situations
Although the goal of every surgeon is to prevent wound infections, they will arise. Treatment is individualized to the patient, the wound, and the nature of the infection. The operating surgeon should be made aware of the possibility of infection in the wound and determine the treatment for the wound. Ideally, surgical care should start with meticulous detail to strategies that prevent the development of SSIs in the first place. Preoperatively, attention should be paid to factors like optimization of patient status, proper asepsis, and surgical site preparation. Intraoperatively, adherence to good basic surgical principles of minimal and fine tissue dissection, proper selection of suture materials, and proper wound closure is important. If a SSI sets in, the treatment often involves opening the wound, evacuating pus, and cleansing the wound. The deeper tissues are inspected for integrity and for a deep space infection or source. Dressing changes allow the tissues to granulate, and the wound heals by secondary intention over several weeks. Early/delayed closure of infected wounds is often associated with relapse of infection and wound dehiscence.
The choice of antibiotic depends on 2 factors—the patient and the known or probable infecting microorganism. Patient factors include allergies, hepatic and renal function, severity of disease process, interaction with other medication(s), and age. In women, pregnancy and breastfeeding must be considered. Therapy must be comprehensive and cover all likely pathogens in the context of this clinical setting.
Introduction
Background
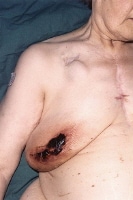
Wound infection due to disturbed coagulopathy. This patient has a pacemaker (visible below right clavicular space) and had previous cardiac surgery (median sternotomy wound visible) for a rheumatic mitral valve disorder, which was replaced. The patient was taking anticoagulants preoperatively. Despite converting to low-molecular weight subcutaneous heparin treatment and establishing normal coagulation studies, she developed a postoperative hematoma with subsequent wound infection. She had the hematoma evacuated and was administered antibiotic treatment as guided by microbiological results, and the wound was left to heal by secondary intention.
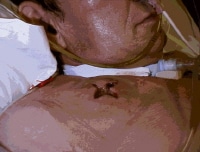
Abscess secondary to a subclavian line.
Hippocrates (Greek physician and surgeon, 460-377 BC), known as the father of medicine, used vinegar to irrigate open wounds and wrapped dressings around wounds to prevent further injury. His teachings remained unchallenged for centuries. Galen (Roman gladiatorial surgeon, 130-200 AD) was first to recognize that pus from wounds inflicted by the gladiators heralded healing (pus bonum et laudabile ["good and commendable pus"]). Unfortunately, this observation was misinterpreted, and the concept of pus preempting wound healing persevered well into the 18th century. The link between pus formation and healing was emphasized so strongly that foreign material was introduced into wounds to promote pus formation-suppuration. The concept of wound healing remained a mystery, as highlighted by the famous saying by Ambroise Paré (French military surgeon, 1510-1590), "I dressed the wound. God healed it."3Pathophysiology
Frequency
United States
International
Mortality/Morbidity
Clinical
History
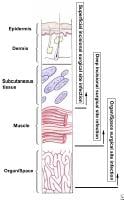
Definitions of surgical site infection (SSI).
Physical
Causes
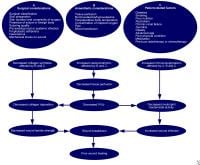
Factors that affect surgical wound healing.
Table 1. Pathogens Commonly Associated with Wound Infections and Frequency of Occurrence11 Pathogen Frequency (%) Staphylococcus aureus 20 Coagulase-negative staphylococci 14 Enterococci 12 Escherichia coli 8 Pseudomonas aeruginosa 8 Enterobacter species 7 Proteus mirabilis 3 Klebsiella pneumoniae 3 Other streptococci 3 Candida albicans 3 Group D streptococci 2 Other gram-positive aerobes 2 Bacteroides fragilis 2
Classification Description Infective Risk (%) Clean (Class I)
No acute inflammation
Closed primarily
Respiratory, gastrointestinal, biliary, and urinary tracts not entered
No break in aseptic technique
Closed drainage used if necessary<2 Clean-contaminated (Class II)
No evidence of infection or major break in aseptic technique
Example: appendectomy<10 Contaminated (Class III)
Gross spillage from gastrointestinal tract
Penetrating traumatic wounds <4>About 20 Dirty-infected (Class IV)
Preoperative perforation of viscera
Penetrating traumatic wounds >4 hoursAbout 40
Differential Diagnoses
Workup
Laboratory Studies
Imaging Studies
Treatment
Medical Care
Operation Expected Pathogens Recommended Antibiotic Orthopedic surgery (including prosthesis insertion), cardiac surgery, neurosurgery, breast surgery, noncardiac thoracic procedures S aureus, coagulase-negative staphylococci Cefazolin 1-2 g Appendectomy, biliary procedures Gram-negative bacilli and anaerobes Cefazolin 1-2 g Colorectal surgery Gram-negative bacilli and anaerobes Cefotetan 1-2 g or cefoxitin 1-2 g plus oral neomycin 1 g and oral erythromycin 1 g (start 19 h preoperatively for 3 doses) Gastroduodenal surgery Gram-negative bacilli and streptococci Cefazolin 1-2 g Vascular surgery S aureus, Staphylococcusepidermidis, gram-negative bacilli Cefazolin 1-2 g Head and neck surgery S aureus, streptococci, anaerobes and streptococci present in an oropharyngeal approach Cefazolin 1-2 g Obstetric and gynecological procedures Gram-negative bacilli, enterococci, anaerobes, group B streptococci Cefazolin 1-2 g Urology procedures Gram-negative bacilli Cefazolin 1-2 g
Table 4. American Society of Anesthesiologists (ASA) Classification of Physical Status23ASA Score Characteristics 1 Normal healthy patient 2 Patient with mild systemic disease 3 Patient with a severe systemic disease that limits activity but is not incapacitating 4 Patient with an incapacitating systemic disease that is a constant threat to life 5 Moribund patient not expected to survive 24 hours with or without operation
Table 5. Predictive Percentage of SSI Occurrence by Wound Type and Risk Index*22At Risk
IndexPredictive Percentage of SSI 0 1.5 1 2.9 2 6.8 3 13.0
Table 6. Data Support RecommendationsCategory Description Category IA Well designed, experimental, strong; recommended (Category I*) clinical or epidemiological best practice; should be studies; adapted by all practices Category IB Some experimental, fairly strong; recommended (Category II*) clinical or epidemiological best practice; should be studies and theoretical grounds; adapted by all practices Category II Fewer scientific supporting data; limited to specific nosocomial (Category III*) problems No recommendation Insufficient scientific personnel judgment for use (Category III*) supporting data 
Abscess secondary to a subclavian line.
Surgical Care
Medication
Antibiotics
Saturday, November 27, 2010
Labels: Wound Infection
Subscribe to:
Post Comments (Atom)
0 comments:
Post a Comment